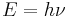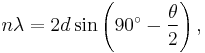Davisson–Germer experiment
| Quantum mechanics | ||||||||||||||||
 |
||||||||||||||||
| Uncertainty principle |
||||||||||||||||
Introduction · Mathematical formulations
|
||||||||||||||||
The Davisson–Germer experiment was a physics experiment conducted by Clinton Davisson and Lester Germer in 1927, which confirmed the de Broglie hypothesis. The de Broglie hypothesis says that particles of matter (such as electrons) have wave properties. This demonstration of wave–particle duality was important historically in the establishment of quantum mechanics and of the Schrödinger equation.
Contents |
History
In 1924 Louis de Broglie presented his thesis concerning the wave-particle, proposing the idea that all matter displayed the wave-particle duality of photons.[1] According to de Broglie, for all matter and for radiation alike, the energy E of the particle was related to the frequency of its associated wave ν, by the Planck relation
and that the momentum of the particle p was related to its wavelength λ by what is now known as the de Broglie relation
where h is Planck's constant.
In 1926, upon knowing the preliminary results of Davisson and Germer, Walter Elsasser remarked that the wave-like nature of matter might be investigated by electron scattering experiments on crystalline solids, as the wave-like nature of X-rays was confirmed through X-ray scattering experiments on crystalline solids.[1][2]
In 1927 at Bell Labs, Clinton Davisson and Lester Germer fired slow moving electrons at a crystalline nickel target.[3] The angular dependence of the reflected electron intensity was measured, and was determined to have the same diffraction pattern as those predicted by Bragg for X-rays. This was also replicated by George Paget Thomson.[1]
The experiment confirmed the de Broglie hypothesis – matter displayed wave-like behaviour. This, in combination with Arthur Compton's experiment, established the wave–particle duality hypothesis, which was a fundamental step in quantum theory.
Experiment
The experiment consisted of firing an electron beam from an electron gun on a nickel crystal at normal incidence (i.e. perpendicular to the surface of the crystal). The electron gun consisted of a heated filament that released thermally excited electrons, which were then accelerated through a potential difference of 54 V, giving them a kinetic energy of 54 eV. An electron detector was placed at an angle θ = 50° to obtain a maximum reading, and measured the number of electrons that were scattered at that particular angle.[1]
According to the de Broglie relation, a beam of 54 eV had a wavelength of 0.165 nm. The experimental outcome was 0.167 nm, which closely matched the predictions of Bragg's law
for n = 1, θ = 50°, and for the spacing of the crystalline planes of nickel (d = 0.091 nm) obtained from previous X-ray scattering experiments on crystalline nickel.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 R. Eisberg, R. Resnick (1985). "Chapter 3 – de Broglie's Postulate—Wavelike Properties of Particles". Quantum Physics: of Atoms, Molecules, Solids, Nuclei, and Particles (2nd ed.). John Wiley & Sons. ISBN 0-471-87373-X.
- ↑ H. Rubin (1995). "Walter M. Elsasser". Biographical Memoirs. 68. National Academy Press. ISBN 0-308-05238-6. http://www.nap.edu/openbook.php?record_id=4990&page=103.
- ↑ C. Davisson, L.H. Germer (1927). "Reflection of electrons by a crystal of nickel". Nature 119: 558–560. doi:10.1038/119558a0.
External links
- R. Nave. "Davisson–Germer Experiment". HyperPhysics. Georgia State University, Physics Departement. http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/davger2.html.